Bdelloid Rotifers: Sex,Take 2
This article is being cross-listed on The Berkeley Science Review. Check out some other really interesting pieces there!
Isaac Newton, one of the most famous physicists to have ever existed, lived to be 84 years old and did so under a celibate promise. Imagine a lifetime without sex. Now imagine tens of millions of years without sex: meet the Bdelloid rotifers (Fig 1). These tiny, female-only, metazoans (0.5 mm in length) are well-known for their asexuality and resilience toward desiccation and ionizing radiation. And while other animals like komodo dragons, some sharks, and stick-insects (Timema stick-insects have reproduced asexually for over 1 million generations!) can asexually reproduce, in most cases during the lack of viable males, it’s incredibly rare to see an animal that exclusively reproduces asexually. Bdelloid (pronounced del●loi●d) rotifers are an “evolutionary scandal” because they do such a thing, completely challenging the sexual reproduction dogma; that is, introducing genetic variation to allow species to adapt to their dynamic environments in addition to mitigating genetic degradation for the benefit of the population. Not having sex isn’t what necessarily makes rotifers scandalous (bacteria don’t have sex and look at how well they’re doing), it’s that they’re complex multicellular organisms who’ve speciated to a degree similar to that of sexually reproducing organisms and who’ve done so asexually.

Normally, sex allows for chromosomal crossover leading to offspring having different combinations of genes from their parents. This gene shuffling mechanism is a major engine for genetic variation in a population by producing gene combinations that can mask deleterious mutations or bring about new positive traits. Despite sex being a successful and prolific mechanism of inheritance, it’s expensive. To name a few of the costs associated with sex we have:
- Offspring inherit only a portion of your genes
- Sex can make you susceptible to predators
- Resources (genetic and behavioral, not just monetary) are often spent trying to seduce mates
- Parasites, viruses, and bacteria take advantage of sex and spread from host to host during the act of sex
The biggest cost of sex is bullet one. If however, you could manage to make a genetic copy of yourself while introducing genetic variation into the population, you could circumvent most of the costs associated with sex. That’s exactly what rotifers seem to be doing: they’re maintaining genetic diversity in the population while avoiding deleterious mutations, and their doing it without sex.
A recent paper by Flot and colleagues delved into a particular bdelloid rotifer genome (the genome of Adineta vaga). These researchers found an incompatible genome structure with that of meiosis: a precursor to sexual reproduction. The reason? The genome of this particular rotifer (and probably others) doesn’t allow pairing of homologous recombination. Interestingly, the allelic gene pairs from this rotifer appear to be distributed in intrachromatic repeats resulting in vastly different chromosomes and a literal structural incompatibility with sexual reproduction (Fig 2). This also means each allele could potentially code for very different proteins in addition to the existence of a nonconventional mechanism for chromosomal pairing, or lack thereof.
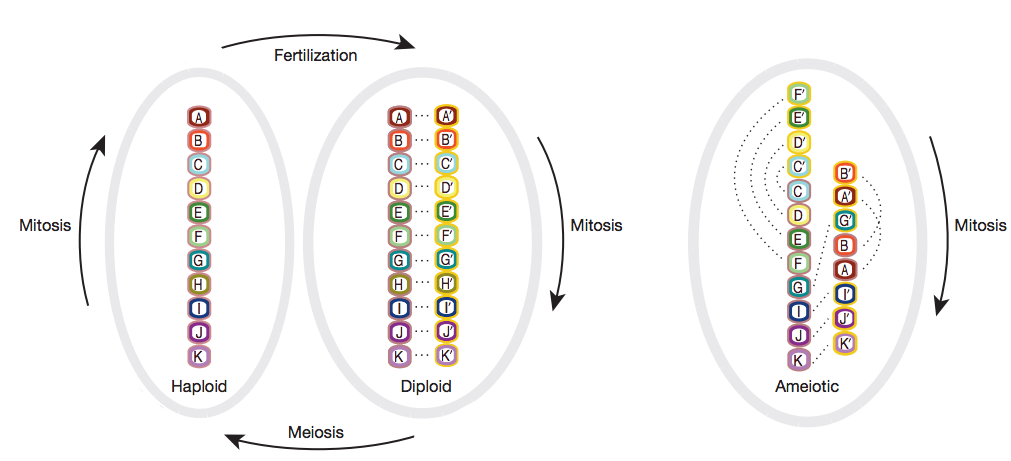
Is there a method to this madness?
The authors describe two primary reasons why rotifers have been successful following an ameiotic lifestyle: horizontal gene transfer and gene conversion. The predominant reason for horizontal gene transfer appears to be for serendipitous bursts of variation in the population. This makes sense if you consider their environment sculpting their Picasso-painting of a genome. Rotifers can withstand long periods of dehydration at any stage in their life cycle. By long, I mean long. They’re capable of surviving desiccation for 9 years, and once rehydrated, they resume normal function. Not only that, Gladyshev and Meselson showed that bdelloid rotifers are highly resistant to ionizing radiation, capable of withstanding doses resulting in hundreds of DNA double-strand breaks per genome with only a ~20% reduction in fertility; at a dose of 3 times less, there’s 99% sterility in the most resilient invertebrate pupa or adult. And since desiccation and ionizing radiation both cause double strand breaks in DNA, incorporation of foreign DNA through horizontal gene transfer and gene conversion aid as a unifying survival mechanism, in addition to bringing in the much needed genetic diversity. According to Flot and colleagues, at least 8% of this rotifer’s genes have been acquired through horizontal gene transfer from other organisms; while this doesn’t sound like much, it’s more than any other known organism. These super bugs are the Peter Petrelli of the natural world: they can do it all (minus “it”).
In conjunction with horizontal gene transfer, gene conversion replaces one allele with another through DNA repair mechanisms. In doing so, some of the progeny will have a deleterious allele overwritten by a beneficial or neutral one, increasing fitness.
Horizontal gene transfer and gene conversion appears to be a formidable option to sexual reproduction, considering how extensive bdelloid rotifers have speciated. However, we can’t rule out the possibility of males existing and having some type of cryptic sex, compatible with the rotifer genome structure, or at the very least a different type of recombination without males altogether: yes, males have never been found and yes these organisms are meiotically incompatible, but absence of evidence is not evidence of absence.
 Alex Padron is a first year graduate student in the UC Berkeley Molecular and Cell Biology program. He is interested in science and in empowering the general audience by making science make sense. You can follow him on Twitter @apadr007 and Google+ gplus.to/apadr007
Alex Padron is a first year graduate student in the UC Berkeley Molecular and Cell Biology program. He is interested in science and in empowering the general audience by making science make sense. You can follow him on Twitter @apadr007 and Google+ gplus.to/apadr007