Just Skin Deep — Your Immune System at the Surface
The skin is the human body’s largest organ. At 1.8 square meters for the average adult, skin covers about as much area as a large closet, and accounts for 12-15% of total body weight. The incredible variation in skin — oily, moist, or dry, exposed to light and cold, or dark and warm — even on an individual, creates unique habitats for the thousands of bacterial and fungal species (called commensal microbiota) that live on our skin. The skin immune system may control skin microbes, but our skin commensals can also educate our immune system. How our skin orchestrates this dialogue with microorganisms and physical insult is integral to its function, and to our health.
The skin is an immunologic organ. There are an estimated 20 billion T cells in human skin — far greater than the number of T cells in the blood — suggesting that immune defense in the skin is a high priority. The interaction among skin microorganisms and the immune system is likely not adversarial most of the time. Interestingly, the incidence of inflammatory skin conditions like atopic dermatitis in children has about doubled in the last thirty years, in parallel with the decreased exposure to microorganisms in early life.

How we understand the dynamic interactions among microbes and immune cells in human skin will have important implications not only for the treatment of autoimmune disorders, skin allergies, and skin malignancies, but also for the creation of better vaccine adjuvants exploiting skin immunity.
Major Players in the Skin Immune Landscape
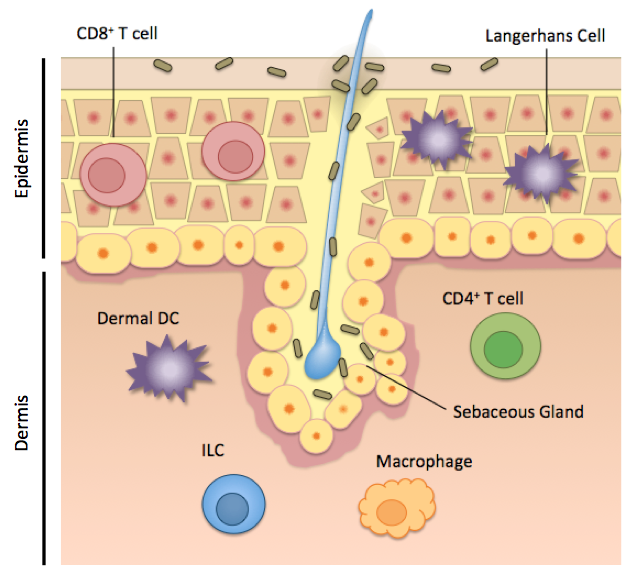
Human skin is home to not only T cells and microorganisms, but also to a diverse group of cells with innate or innate-like functions. These include keratinocytes and Langerhans cells in the epidermis, and dermal dendritic cells, macrophages, and innate lymphoid cells in the dermis (Figure 1). While much work up to this point has elucidated the individual roles of these cells, describing how the total interactions among these cells maintain health or dysfunction in disease, will shape the ongoing skin research dialogue.
Keratinocytes are the major cell type that makes up the epidermis, and while not of myeloid or lymphoid origin, play important immune defense roles. Keratinocytes produce some antimicrobial peptides that control resident microorganisms on the skin, and also express some pattern recognition receptors, like toll-like receptors, that allow the activation of this cell type upon pathogen recognition or cell damage. Keratinocytes can produce pro-inflammatory cytokines to activate its neighbor, the Langerhans cell.
Langerhans cells are the first immune cells that any skin-invading pathogen or commensal will come in contact with, and are also activated in response to cell damage and UV light. Langerhans cells are the primary antigen-presenting cell in the epidermis, and are identified by the receptor Langerin, and the lipid-presenting molecule CD1a in humans. CD1a is highly abundant in human skin on Langerhans cells and dermal dendritic cells, and has been shown to bind skin oils and engage reactive T cells. Mice lack this antigen-presentation molecule, however, and the functional significance of CD1a in human skin is continuing to be explored by scientists.
Figure 1. Immune cells in the skin occupy distinct locations and functional roles. Figure created by Rachel Cotton, adapted from Pasparakis M et al, 2014
Dermal dendritic cells. A skin dendritic cell “samples” its surroundings, picking up antigen from a damaged cell, a pathogen, or a commensal microorganism, and then traveling to the skin-draining lymph node. There, the dendritic cell activates and instructs T lymphocytes to come back to the skin and carry out functions like secreting cytokines. There are several distinct dendritic cell subsets in the epidermis and dermis, but the DCs that seem to have the best-defined roles so far, are the CD141+ DCs and CD1c+ DCs. Interestingly, these subsets are relatively functionally equivalent in humans and in mice. The CD141+ DCs in humans, are best at migrating and presenting antigen to T cells in the draining lymph node. These DCs are also good “cross-presenters” meaning that these cells can also process and present exogenous antigen to CD8 T cells, which are abundant in the epidermis. The CD1c+ dendritic cell subset rather is better at “turning on” CD4+ T helper cells in the dermis. The CD1c+ DCs can produce a broad range of cytokines that fine-tune the T cell immune response in the skin. These cells have also been reported to induce T regulatory cells, which would help maintain tolerance to commensal skin microorganisms. Dermal dendritic cells are the key “middle men” between sensing commensal microbes and instructing a T cell response.
Skin-resident T cells. The skin is home to roughly 20 billion T cells, making it the largest reservoirs of T cells in the body. The hallmark of T lymphocytes is their specificity and memory for a given antigen. While the skin dendritic cell populations are generally functionally equivalent between humans and mice, skin T cell populations differ considerably between mice and man, limiting the conclusions we can draw from mouse studies.
In human skin, T cells are described on several metrics: expression of CD4 (T helper cells) or CD8 (Cytotoxic T lymphocytes), if they stay put in the skin or migrate to and from the skin, what cytokines they produce, their T cell receptor (innate-like, αβ or γδ), and how they behave during health and disease. Greater than 95% percent of T cells in the skin have a “memory” phenotype, meaning that these cells have already experienced their cognate antigen, and are poised to rapidly respond to that antigen again. On the other hand, this site-specific memory means that misdirected T cell responses during autoimmune disease cause skin lesions that, even after treatment, recur in the same place.
CD8+ T cells, also known as cytotoxic T lymphocytes (Tc), live almost exclusively in the human epidermis, presumably to rapidly respond to viral infection or tissue damage. CD4+ T cells, called T helper (Th) cells, reside predominantly in the dermis and carry out a variety of effector functions. Both CD4+ and CD8+ T cells can produce key cytokines that mediate skin health or disease, like IL-17 (produced by Th17 or Tc17 cells), IL-22 (produced by Th22 or Tc22 cells), IFNγ (produced by Th1 cells), and IL-10, among others. The cells that exclusively produce IL-22 (Tc22 and Th22 cells) are unique to humans, and are hugely expanded in psoriasis. This overproduction of IL-22 activates epithelial cells and contributes to the red and flaky skin characteristic of psoriasis. A population of CD4+ cells called T regulatory cells (Tregs) on the other hand, help to limit inflammation in the skin, by the production of IL-10 and TGF-β.
The beauty of T lymphocytes is their specificity and memory for a given antigen. Interestingly, the T cell receptor repertoire of human skin is restricted — meaning that there are fewer unique T cell receptors — relative to blood, despite the large cell number. A major question that remains is the cognate antigens for these cells. Do these cells respond to a protein or lipid from a commensal microorganism? A self antigen? Recently, αβ T cells that recognize the human lipid antigen presenting molecule CD1a and some skin oils were found to home to skin, and are relatively common events in human skin and blood. These cells point to a new mechanism of antigen recognition and skin immunity which is still being explored. Other innate-like T cells that have a defined or restricted TCR and antigen, like Natural Killer T cells, are also found in human skin, but their function and roles in skin homeostasis and inflammation are continuing to be understood.
Innate lymphoid cells (ILCs) are cells of lymphoid origin like T cells, but they lack a T cell receptor and therefore lack the ability to respond to a specific cognate antigen. ILCs are rare, but potent, cells residing in the dermis and subcutaneous fat that orchestrate tissue homeostasis and inflammation. ILCs are divided into three groups based on what cytokines they produce, parallel to how CD4 T helper cells are classified. Group 1 ILCs preferentially produce the pro-inflammatory cytokines TNFa and IFNy, whereas group 2 ILCs produce the type 2 cytokines IL-4, IL-5, and IL-13 and are involved in allergy and immunity to helminths. Group 2 ILCs are the predominant ILCs in the human dermis. Group 3 ILCs produce IL-17 and IL-22 and are enriched in psoriatic skin compared to normal skin, suggesting that they play a role in pathology. It was recently demonstrated in the gut however, that group 3 ILCs are responsible for the negative selection of T lymphocytes that recognize gut commensal microbes. The distinct roles of ILCs in human skin at homeostasis and in skin diseases are active areas of research.
The Skin Microbiome and Immunity
The incredible variation in skin at different sites on the human body creates unique habitats for the over 1000 bacterial and fungal species – called commensal microbiota – that live on our skin. Human skin commensals have been extensively characterized in recent years by 16s rRNA sequencing with the Human Microbiome Project and human skin sites surveys, revealing striking site-specific microbial signatures depending on physical factors like oiliness, dampness, and exposure to sunlight. In other words, skin commensals on the bottom of your foot are a completely different population from the commensals on your forearm, which look completely different from the microbiota populations on your face.

Commensal microbes in general have been proposed to promote skin immunity by inducing a basal level of immune activation, mediated by IL-1, that protects against other infections. Indeed, skin studies in mouse models have demonstrated that skin microbiota can modulate skin-resident T cell populations. For example, Staphylococcus epidermidis (S. epi) colonization of mouse epidermis leads to the accumulation of Tc17 T cells in the epidermis that are protective against fungal infection. However, injection of this S. epi into the dermis results in inflammation and a visible wound, underscoring that for the mammalian skin immune system, location of a microbe matters.
In humans, changes in skin microbiota also track with skin diseases. For example, Staphylococcus aureus has been associated with atopic dermatitis flares in children. It is tempting to speculate that skin pathologies that show a predilection for certain tissue sites, like atopic dermatitis in the creases of the elbows and knees, are in part driven by the unique microbial signature at that site, or a shift in the population at that site.
Conclusions
The skin is packed with immune cells, in constant dialogue with commensal microorganisms that live on us and in us. There is still a lot to be understood about how specific microbes shape the skin immune system, how and why we are “tolerant” to our skin microorganisms in health, and how immune responses to these microorganisms contribute to skin disease.
Understanding how immune cells function diverse skin environments with distinct microbial communities will have implications for the treatment of inflammatory skin diseases, skin cancers, and the creation of vaccine adjuvants that take advantage of the unique qualities of human skin-resident immunity.
For more updates on the interactions among skin bacteria and your immune system from dermatologists, immunologists, and microbiologists, check out the Skin Microbiome Blog at skinmicrobiome.wordpress.com.

Rachel Cotton is a PhD student in the Immunology Program at Harvard, and is interested in global health and science policy. Her past research includes how parasites modulate skin immunity. Follow her on Twitter @RachCotton.